E-Etching: Art, Science, Craft

Have No Fear! Electro etching (or e-etch for short) is way less prone to give you an electric shock compared to your kitchen and household appliances. The etching process is performed with 0.5 to 1.5 Volts which is not even anywhere near the strength of your flashlight batteries. Instead, e-etch provides an elegant and in many ways superior alternative to etch copper, zinc and iron intaglio plates. Since some mighty fine and detailed articles have already been written on the process, the basics, and details of electro etching, I feel there is no point to regurgitate these here: instead, I will provide you the references to the relevant websites and publications for your further theoretical information and perusal.
My interest and approach to e-etching comes from my background as a technical engineer – the versatility of the process paired with convincingly superior etching results (well… sometimes, as I will explain a bit later) have meanwhile made me to totally turn to e-etching for intaglio printmaking work. After an autodidactic start some four years ago, I have attended artist in residence workshops with experts on e-etching to gain experience and learn the “tricks of the trade”. For anyone interested in starting e-etching, I would really recommend booking a workshop to become acquainted with the basics and to find out, whether this process is something you like and might adopt for your artwork. A hands-on start-up guidance will allow a swift initiation and immersion into the process and will avoid frustration when fine details might have been overlooked.

The next item in the setup is an adjustable AC to DC transformer which can handle a range of 0 to 10 V output. The higher the amperage (given in “A”) the better as this will decide how large of a plate you will be able to etch eventually. Quite suitable transformers you will be able to buy for some 60 to 70 USD. The photo shows the transformer and the small test tank already with anode (the copper plate to be etched) and the cathode which is either the same metal as the anode or a stainless-steel grid or mesh. Anode and cathode should ideally be approximately 6 cm (2 ½ inches) apart.
All you need now to start etching is the electrolyte which will electrically connect the two plates (anode and cathode).
The type of metal of your plate to be etched determines the electrolyte you will need: the blue Copper Sulfate (CuSO4) for copper, the white Zinc Sulfate (ZnSO4) for zinc and the green Iron Sulfate (FeSO4) for iron.
All of the before mentioned sulfates can be bought in crystal form and have to be dissolved in de-ionized or distilled water to avoid potential and unwanted reactions with other chemicals/minerals in the water (e.g. chlorine). The concentration (the “strength”) of the electrolytes will determine the etching speed – generally, the higher the concentration, the faster the etching will progress. When mixing up the electrolytes take care to observe the SDS’s (Safety Data Sheets); once the electrolytes have been prepared, you can store them in plastic jerry cans. “Advantage One” of electro etching is that the electrolytes are not consumed or weakened through their use and there will be no precipitations that will require special disposal.

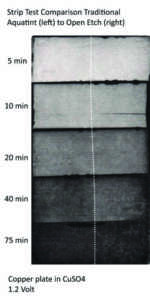
Over the past years, I have seen good results e-etching copper, zinc and iron. Copper seems to respond best to this process. Iron and non-alloyed steels can also be electro etched with very satisfactory results. There are some opinions and publications around that aluminum can also be electro etched, but in my experience the results were suboptimal. With a recent change in the material composition for zinc, we have experienced some challenges not to say disappointments: the metallurgical industry has tried to improve the quality of zinc plates by adding small amounts of titanium and copper to the plates (now called “TitanZinc” in line with standards EN 988 and ASTM B69) to increase stability and corrosion resistance – this was certainly appreciated by the construction industry but an increased corrosion resistance is not quite what you want for etching. While line etches pose no problem, the open etch “e-tinting” has become quite a challenge.
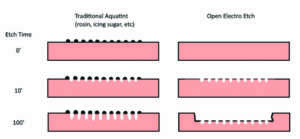
Over the past years, I have seen good results e-etching copper, zinc and iron. Copper seems to respond best to this process. Iron and non-alloyed steels can also be electro etched with very satisfactory results. There are some opinions and publications around that aluminum can also be electro etched, but in my experience the results were suboptimal. With a recent change in the material composition for zinc, we have experienced some challenges not to say disappointments: the metallurgical industry has tried to improve the quality of zinc plates by adding small amounts of titanium and copper to the plates (now called “TitanZinc” in line with standards EN 988 and ASTM B69) to increase stability and corrosion resistance – this was certainly appreciated by the construction industry but an increased corrosion resistance is not quite what you want for etching. While line etches pose no problem, the open etch “e-tinting” has become quite a challenge.
As mentioned, my technical background has led me to a systematic approach to e-etching with running numerous (over 200) test strips for copper, zinc, and iron varying electrolyte concentrations, applied etching voltages and distances between anode and cathode. Results have been analyzed not only by test prints but also in tabular form and under a reflecting light microscope where finally the “Advantage Three” was confirmed, that the lines actually etch straight vertically into the metal with no erosion (or corrosion) to the sides.
I would be glad to share more details of my tests and assessments, please contact me directly with your questions – or suggestions, as the case may be! [email protected] www.keicie.com
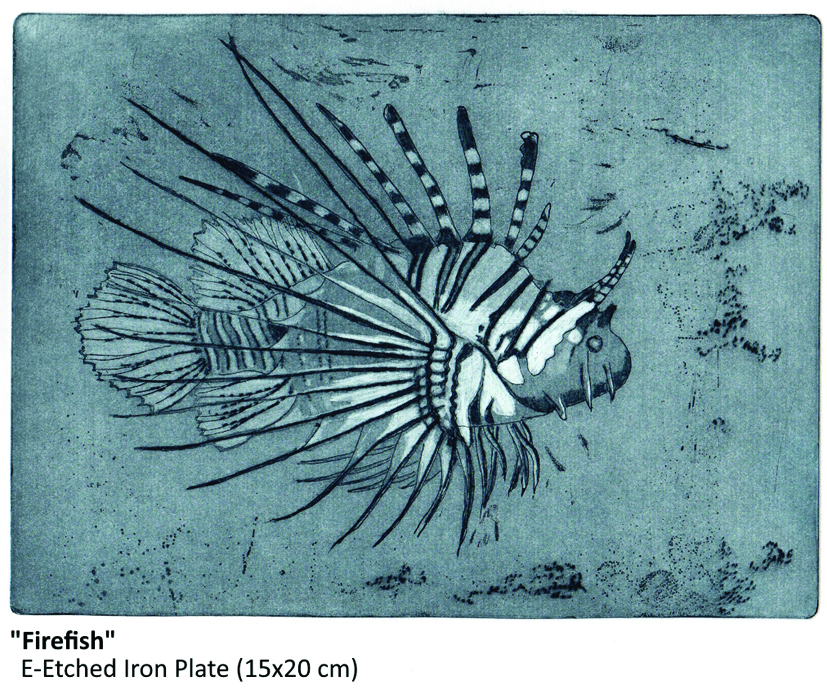
In closing some samples of my e-etched intaglio prints:


Literature References:
Baldwin, Andrew: www.printmakingstudio.co.uk (Baldwin Intaglio Ground (BIG) with many very useful videos con-cerning the application of this etching ground)
Behr, Omri and Behr, Marion: CHEMTECH, April 1991, 21(4), 210-215 Copyright 1991 American Chemical Society)
Behr, Omri and Behr, Marion: Etching and Tone Creation Using Low-Voltage Anodic Electrolysis; LE-ONARDO 25 #1 (1993)
Bossmann, Katharina et al: An Optimized Nontoxic Electrolytic Etching Procedure for Fine Art Print-making; Leonardo (2021) 54 (4): 427–432; https://doi.org/10.1162/leon_a_02000
*Please note that Mrs Bossmann reports the use of the not-so-common copper-heptahydrate (CuSO4.7H2O) compared to the use of copper pentahydrate (CuSO4.5H2O): the suggested amount used in her experiments (62.5 g/l) for the heptahydrate electrolyte equates to 71.5 g/l of pentahydrate to achieve the equivalent concentration of CuSO4.
Crujera, Alfonso: www.en.crujera.com/publications/articles/electro-etching-made-easy (detailed step-by-step description of the electro etching process with many helpful photos)
Green, Cedric: www.greenart.info/galvetch (Cedric refers to the process as “galvanic etch”)
Semenoff, Nik et al: Using Dry Copier Toners in Intaglio and Electro-etching of Metal Plates
Published in LEONARDO, Vol. 24, No. 4, 1991 – received May 1989















